AlCl3 Molecular Geometry - Unpacking Its Shape
Have you ever wondered how tiny bits of matter arrange themselves, shaping everything around us? It's pretty fascinating, actually. We often hear about molecules, but what gives them their unique forms? Today, we are going to talk about a rather interesting compound, aluminum chloride, which chemists often call AlCl3. Its particular shape, or molecular geometry, holds some rather surprising details, especially when it is not in its usual solid state.
Figuring out the exact spatial arrangement of atoms within a molecule is, you know, a bit like trying to picture a tiny, invisible sculpture. For something like AlCl3, knowing its geometry helps us figure out how it might behave or react with other things. It’s not just a flat drawing on paper; these atoms truly exist in a three-dimensional space, and their positions really matter.
So, we will look at how scientists figure out these shapes, starting with something called a Lewis structure, which is basically a map of the electrons. From there, we can sort of, you know, predict the molecule's overall shape. It's a key step in understanding many chemical reactions and, as a matter of fact, why some compounds act the way they do.
- Chatgpt Plus Subscription Price Iran
- Grace Sward Nude
- Dylan Dreyers Family Situation
- How To Subscribe To Chatgpt Plus In Iran
- Openai Chatgpt Subscription Iran Payment
Table of Contents
- What is AlCl3 Molecular Geometry, Anyway?
- Drawing the Lewis Structure for AlCl3 - A First Step
- How Do Electron Domains Shape AlCl3 Molecular Geometry?
- What is the Molecular Geometry of AlCl3 in its Simple Form?
- AlCl3 Molecular Geometry When It Dimerizes
- The Bridging Atoms in Al2Cl6 and Their Impact on AlCl3 Molecular Geometry
- Why Does AlCl3 Molecular Geometry Matter for Catalysis?
- How Different States Affect AlCl3 Molecular Geometry
What is AlCl3 Molecular Geometry, Anyway?
When we talk about something's molecular geometry, we are really just describing the specific way its atoms are arranged in three-dimensional space. Think of it like building a tiny model with balls and sticks. The shape that model takes is its geometry. For AlCl3, this shape isn't just some random thing; it has a lot to say about how the compound behaves, how it might connect with other chemical pieces, or even how it might, you know, dissolve in something. It’s pretty central to its identity, you could say. Every single molecule, whether it's water or something more complicated, has its own characteristic shape, and these shapes are what give them their individual traits. This arrangement of parts, you know, influences everything from how it smells to how it reacts, so it’s a rather big deal in the world of tiny things.
So, why bother with this geometry business for AlCl3? Well, it turns out that the particular way aluminum and chlorine atoms sit next to each other in this compound really influences its actions. For instance, its shape plays a part in how it functions as a helper in industrial processes, a role we will touch on a little later. It’s like knowing the precise form of a key helps you understand which lock it will open. Without knowing its shape, our ability to predict its usefulness or its interactions would be, you know, pretty limited. This kind of spatial arrangement is a foundational idea in chemistry, and for AlCl3, it has some quite distinct characteristics that are worth exploring, actually.
Drawing the Lewis Structure for AlCl3 - A First Step
To begin figuring out a molecule's shape, chemists often start by drawing what's called a Lewis structure. This is basically a simple drawing that shows all the valence electrons, which are the outermost electrons, and how they are shared or transferred between atoms. For AlCl3, you place the aluminum atom, which is Al, right in the middle, because it is the least electronegative element here. Then, you put the three chlorine atoms, Cl, around it. Each line drawn between the atoms represents a shared pair of electrons, forming a chemical bond. You also draw dots around the chlorine atoms to show their unshared electron pairs, or what we call lone pairs. This drawing is, in a way, a blueprint of the molecule's connections.
- Teach Me First Honeytoon Free
- George Reeves Christopher Reeves Related
- Openai Chatgpt Plus Payment Iran
- Honey Toon Teach Me First For Free
- Are David And Hilary Married
Now, when you draw this for AlCl3, you will see something rather interesting about the central aluminum atom. While most atoms try to get eight electrons around them in bonds or lone pairs, a rule often called the octet rule, aluminum in AlCl3 ends up with only six electrons. It has three bonds, and each bond has two electrons, so that makes six. This makes the aluminum atom, you know, a bit "electron poor" or "electron deficient." This particular electron count, or rather, the lack of a full octet, is actually a very important piece of information. It explains a lot about AlCl3's behavior, and why it acts the way it does in various situations. It is, basically, a key characteristic that sets it apart.
How Do Electron Domains Shape AlCl3 Molecular Geometry?
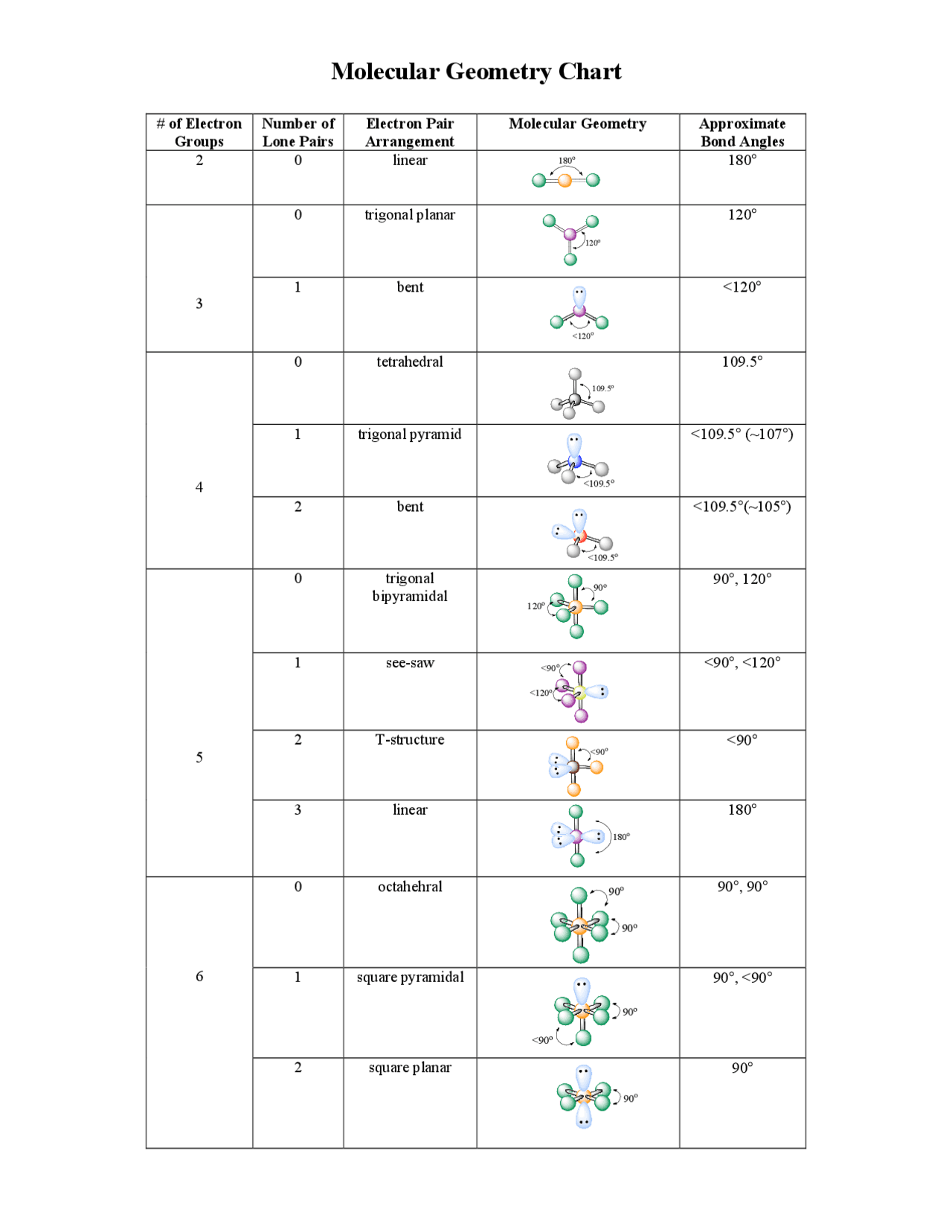
Once you have a Lewis structure, the next step in figuring out the AlCl3 molecular geometry involves something called "electron domains." An electron domain is simply a spot where electrons are located around a central atom. This could be a single bond, a double bond, a triple bond, or even a lone pair of electrons. The main idea here is that these electron domains, because they are all negatively charged, try to get as far away from each other as they possibly can. It is a bit like magnets pushing each other apart, you know, to find the most comfortable spacing. This push-and-pull determines the overall arrangement of electron groups around the central atom.
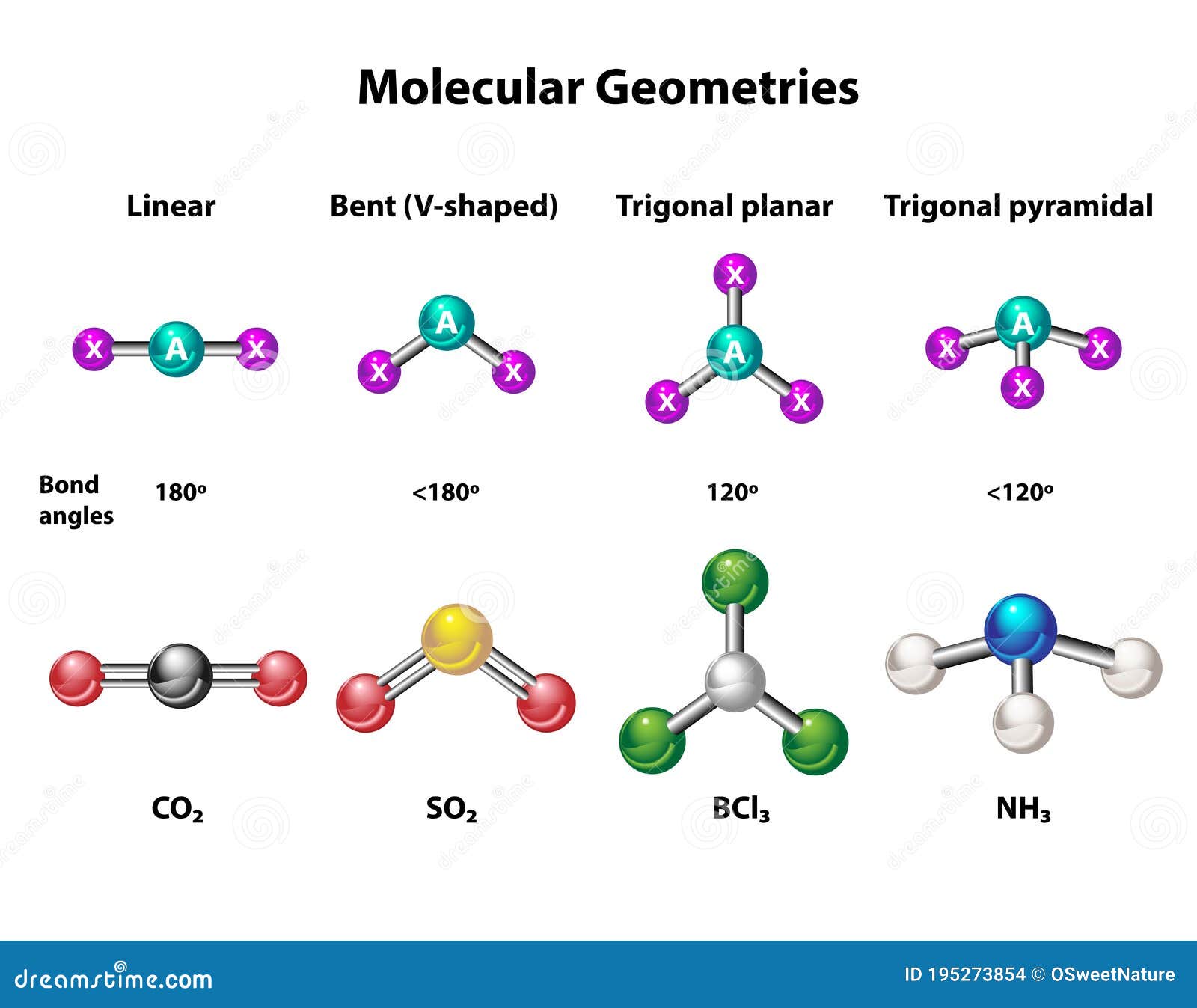
For our AlCl3 example, the central aluminum atom has three bonds connecting it to three chlorine atoms. There are no lone pairs of electrons on the aluminum atom itself. So, this means the aluminum atom has three electron domains around it, all of which are bonding pairs. Since these three electron domains want to be as far apart as possible, they arrange themselves in a flat, triangular shape. This arrangement is called "trigonal planar." It is a fundamental shape that comes up quite often when you have three groups around a central atom with no lone pairs. This simple principle, you know, helps predict the initial spatial layout of the electrons around the aluminum.
What is the Molecular Geometry of AlCl3 in its Simple Form?
So, we just talked about electron domain geometry, which considers all electron groups, including lone pairs. Now, molecular geometry is a bit different; it only considers the positions of the atoms themselves. If the central atom has no lone pairs, then the electron domain geometry and the molecular geometry will be the same. And for AlCl3, as we just saw, the central aluminum atom does not have any lone pairs of electrons. It only has the three bonds reaching out to the chlorine atoms. This means that its molecular geometry will also be, you know, trigonal planar. It is a very flat shape, almost like a perfect triangle with the aluminum in the middle and the three chlorines at the points.
Imagine a tiny, perfectly flat triangle, perhaps a bit like a peace sign, with the aluminum right in the very center and a chlorine atom at each of the three corners. The angles between the bonds in this setup are all 120 degrees, which is, you know, the widest they can get while still staying in that flat arrangement. This specific shape is what you would expect for a single AlCl3 molecule when it is, say, in a solid crystal where it exists as individual units, or when it is very diluted. This simple, flat structure is, basically, the standard way we picture AlCl3 when it is on its own, not interacting too much with other AlCl3 pieces.
AlCl3 Molecular Geometry When It Dimerizes
Now, here is where the story of AlCl3 molecular geometry gets a little more interesting, actually. While AlCl3 exists as individual, trigonal planar molecules in its solid form, something quite different happens when it is in the molten (melted) state or as a gas. In these conditions, two AlCl3 units decide to join together, forming a larger structure. This process is called "dimerization," and the resulting combined molecule is known as a "dimer." For aluminum chloride, this dimer has the chemical formula Al2Cl6. It is, you know, two of the smaller pieces becoming one bigger piece. This change is pretty important for how it behaves in these different physical states.
The main reason AlCl3 forms this dimer goes back to that electron deficiency we talked about earlier. Remember how the aluminum atom in a single AlCl3 molecule only had six electrons around it? Well, atoms generally prefer to have eight electrons to feel more stable. So, to fix this, two AlCl3 molecules come together, and they share some of their chlorine atoms. This sharing allows each aluminum atom to gain two more electrons, bringing its total to eight, which is, you know, a much more stable arrangement for it. This desire for stability drives the formation of the Al2Cl6 dimer, completely changing the AlCl3 molecular geometry for each aluminum atom involved.
The Bridging Atoms in Al2Cl6 and Their Impact on AlCl3 Molecular Geometry
In the Al2Cl6 dimer, the structure is quite clever. You have two aluminum atoms, and they are linked by two chlorine atoms that act as "bridges." Imagine two people standing side-by-side, holding hands across to each other. Those clasped hands are, sort of, like the bridging chlorine atoms. Each aluminum atom is also connected to two other chlorine atoms that are not bridging; these are called "terminal" chlorines. So, each aluminum atom in the dimer is actually bonded to four chlorine atoms in total: two terminal ones and two bridging ones. This is a very different setup from the simple AlCl3 molecule, and it profoundly changes the AlCl3 molecular geometry around each aluminum.
Because each aluminum atom in the Al2Cl6 dimer is now connected to four other atoms, its electron domain geometry and molecular geometry both shift. Instead of the flat, trigonal planar shape, each aluminum atom now adopts a "tetrahedral" arrangement. A tetrahedral shape is, you know, like a pyramid with a triangular base, or perhaps a bit like a tripod with four legs spreading out in three dimensions. This means that the angles around each aluminum atom are no longer 120 degrees, but closer to 109.5 degrees, which is what you see in a perfect tetrahedron. This change in shape allows each aluminum atom to achieve that desired eight-electron count, making the dimer a much more stable form in the gas and molten phases. It's a rather significant change from the monomer, you know, in terms of its overall shape and electron count.
Why Does AlCl3 Molecular Geometry Matter for Catalysis?
The source text mentions that aluminum chloride, AlCl3, is used as a catalyst in various industrial reactions. So, what does that mean, and how does its shape play a part? A catalyst is, basically, something that helps a chemical reaction happen faster without being used up itself. AlCl3 is known as a "Lewis acid," which means it is very good at accepting a pair of electrons from another molecule. This ability to accept electrons is directly tied to that electron deficiency we talked about earlier, where the aluminum atom only has six electrons around it in the simple AlCl3 form. It is, you know, always looking for more electrons to complete its set.
The trigonal planar AlCl3 molecular geometry of the monomer, or even the tetrahedral arrangement in the dimer, leaves a spot open for other molecules to interact with the aluminum atom. That flat, open shape of the monomer, for instance, makes it relatively easy for another molecule to approach the aluminum atom and donate a pair of electrons to it. When it accepts those electrons, it forms a temporary bond, which can then help break or form other bonds in the reacting molecules, speeding up the process. So, its shape, and the fact that it is, you know, a bit electron-hungry, are exactly what make it so useful as a helper in these chemical transformations. It is, essentially, its willingness to accept electrons that makes it a good catalyst.
How Different States Affect AlCl3 Molecular Geometry
It is pretty interesting how the physical state of aluminum chloride really influences its AlCl3 molecular geometry. In its solid form, AlCl3 exists as a network of individual AlCl3 units, each maintaining that trigonal planar shape we discussed. The forces holding these individual units together in the solid are not as strong as the bonds within the molecules themselves, so they keep their distinct, flat shapes. However, when you heat it up and it becomes a liquid (molten) or turns into a gas, the story changes quite a bit. This is where the dimerization happens, forming the Al2Cl6 structure. The change in state, you know, provides the energy for this structural transformation.
In the molten and gas phases, the individual AlCl3 molecules have enough energy to move around more freely. This increased movement and energy allows them to rearrange themselves into the more stable Al2Cl6 dimer. This means that if you are working with aluminum chloride in a lab and it is melted or in a gaseous form, you are actually dealing with the Al2Cl6 dimer, where each aluminum atom has a tetrahedral arrangement, not the simple trigonal planar AlCl3 monomer. This difference is, you know, quite important for chemists because the way a compound behaves can be very different depending on its actual structure at that moment. So, the environment, basically, dictates the geometry.
- Gloria Torres Of Only Fans
- Buy Chatgpt Plus In Iran
- Charlize Therons Personal Life
- Teach Me First Comic Honey
- Lara Storm Erome
Molecular Geometry Structure of Elements Stock Vector - Illustration of
Spatial And Electron Pair Geometry Molecular Geometry - vrogue.co
Solved Please note that "geometry" refers to the molecular | Chegg.com